Sanofi termina l'accordo sulla licenza del farmaco dopo le obiezioni della FTC statunitense -11 dicembre 2023 alle 22:33 | MarketScreener

Sanofi Italia on X: "La nostra ricerca sulle malattie rare non si ferma: sono 39 le presentazioni che terremo in questi giorni al @WORLDSymposia 2022 a San Francisco. Tra queste, anche nuovi
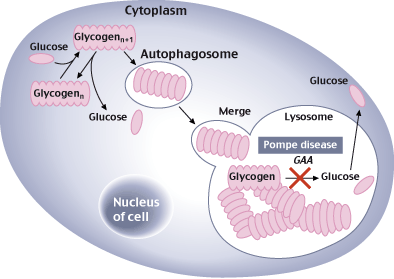
FDA aprueba Nexviazyme® de Sanofi Genzyme, una nueva e importante opción de tratamiento para la enfermedad de Pompe